Cleavage of C–O bond in ethers :

`=>` Ethers are the least reactive of the functional groups.
`=>` The cleavage of `color{red}(C-O)` bond in ethers takes place under drastic conditions with excess of hydrogen halides.
`=>` The reaction of dialkyl ether gives two alkyl halide molecules.
`color{red}(R- O - R + HX → RX + R - OH)`
`color{red}(R - OH +HX → R- X +H_2O)`
`=>` Alkyl aryl ethers are cleaved at the alkyl-oxygen bond due to the more stable aryl-oxygen bond.
● The reaction yields phenol and alkyl halide. See fig.1.
`text(Note :)` Ethers with two different alkyl groups are also cleaved in the same manner.
`color{red}(R -O-R' +HX → R-X +R' - OH)`
`=>` The order of reactivity of hydrogen halides is as follows : `color{red}(HI > HBr > HCl)`.
● The cleavage of ethers takes place with concentrated `color{red}(HI)` or `color{red}(HBr)` at high temperature. See Mechanism as shown in image.
● However, when one of the alkyl group is a tertiary group, the halide formed is a tertiary halide.
`color{red}(CH_3 - underset (underset(CH_3)(|)) overset( overset(CH_3)(|))C-O-CH_3+HI → CH_3OH+CH_3-underset( underset(CH_3)(|)) overset(overset(CH_3)(|))C-I)`
● It is because in step 2 of the reaction, the departure of leaving group `color{red}((HO–CH_3))` creates a more stable carbocation `color{red}([(CH_3)_3C^+])` and the reaction follows `color{red}(S_N 1)` mechanism. See fig.2.
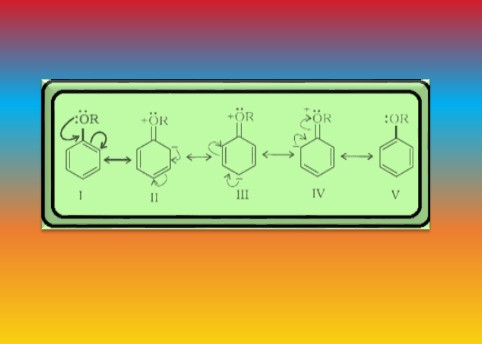
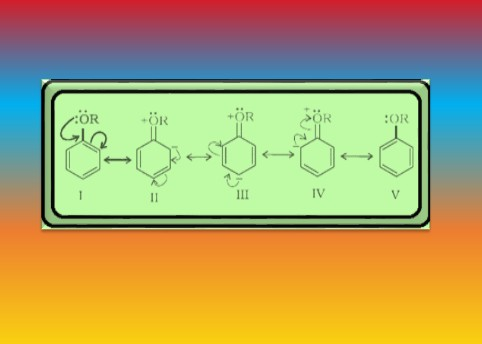
`=>` In case of anisole, methylphenyl oxonium ion, `color{red}(C_6H_5 - underset (underset(H)(|)) overset(+)O-CH_3)` is formed by protonation of ether. The bond between `color{red}(O–CH_3)` is weaker than the bond between `color{red}(O–C_6H_5)` because the carbon of phenyl group is `color{red}(sp^2)` hybridised and there is a partial double bond character.
● Therefore, the attack by `color{red}(I^–)` ion breaks `color{red}(O–CH_3)` bond to form `color{red}(CH_3I)`.
● Phenols do not react further to give halides because the `color{red}(sp^2)` hybridised carbon of phenol cannot undergo nucleophilic substitution reaction needed for conversion to the halide.
`=>` The cleavage of `color{red}(C-O)` bond in ethers takes place under drastic conditions with excess of hydrogen halides.
`=>` The reaction of dialkyl ether gives two alkyl halide molecules.
`color{red}(R- O - R + HX → RX + R - OH)`
`color{red}(R - OH +HX → R- X +H_2O)`
`=>` Alkyl aryl ethers are cleaved at the alkyl-oxygen bond due to the more stable aryl-oxygen bond.
● The reaction yields phenol and alkyl halide. See fig.1.
`text(Note :)` Ethers with two different alkyl groups are also cleaved in the same manner.
`color{red}(R -O-R' +HX → R-X +R' - OH)`
`=>` The order of reactivity of hydrogen halides is as follows : `color{red}(HI > HBr > HCl)`.
● The cleavage of ethers takes place with concentrated `color{red}(HI)` or `color{red}(HBr)` at high temperature. See Mechanism as shown in image.
● However, when one of the alkyl group is a tertiary group, the halide formed is a tertiary halide.
`color{red}(CH_3 - underset (underset(CH_3)(|)) overset( overset(CH_3)(|))C-O-CH_3+HI → CH_3OH+CH_3-underset( underset(CH_3)(|)) overset(overset(CH_3)(|))C-I)`
● It is because in step 2 of the reaction, the departure of leaving group `color{red}((HO–CH_3))` creates a more stable carbocation `color{red}([(CH_3)_3C^+])` and the reaction follows `color{red}(S_N 1)` mechanism. See fig.2.
`=>` In case of anisole, methylphenyl oxonium ion, `color{red}(C_6H_5 - underset (underset(H)(|)) overset(+)O-CH_3)` is formed by protonation of ether. The bond between `color{red}(O–CH_3)` is weaker than the bond between `color{red}(O–C_6H_5)` because the carbon of phenyl group is `color{red}(sp^2)` hybridised and there is a partial double bond character.
● Therefore, the attack by `color{red}(I^–)` ion breaks `color{red}(O–CH_3)` bond to form `color{red}(CH_3I)`.
● Phenols do not react further to give halides because the `color{red}(sp^2)` hybridised carbon of phenol cannot undergo nucleophilic substitution reaction needed for conversion to the halide.